Abstract
The efficacy of lapatinib versus trastuzumab combined with taxanes in the first-line setting of human epidermal growth factor receptor 2 (HER2) -positive metastatic breast cancer (BC) is unknown.
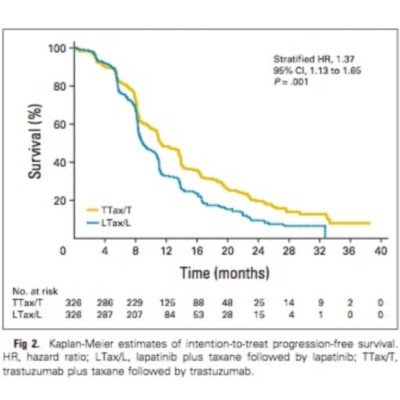
The MA.31 trial compared a combination of first-line anti-HER2 therapy (lapatinib or trastuzumab) and taxane therapy for 24 weeks, followed by the same anti-HER2 monotherapy until progression. Stratification was by prior (neo)adjuvant anti-HER2 therapy, prior (neo)adjuvant taxane, planned taxane, and liver metastases. The primary end point was intention-to-treat (ITT) progression-free survival (PFS), defined as time from random assignment to progression by RECIST (version 1.0) criteria, or death for patients with locally assessed HER2-positive tumors. The primary test statistic was a stratified log-rank test for noninferiority. PFS was also assessed for patients with centrally confirmed HER2-positive tumors.
From July 17, 2008, to December 1, 2011, 652 patients were accrued from 21 countries, resulting in 537 patients with centrally confirmed HER2-positive tumors. Median follow-up was 21.5 months. Median ITT PFS was 9.0 months with lapatinib and 11.3 months with trastuzumab. By ITT analysis, PFS was inferior for lapatinib compared with trastuzumab, with a stratified hazard ratio (HR) of 1.37 (95% CI, 1.13 to 1.65; P = .001). In patients with centrally confirmed HER2-positive tumors, median PFS was 9.1 months with lapatinib and 13.6 months with trastuzumab (HR, 1.48; 95% CI, 1.20 to 1.83; P < .001). More grade 3 or 4 diarrhea and rash were observed with lapatinib (P < .001). PFS results were supported by the secondary end point of overall survival, with an ITT HR of 1.28 (95% CI, 0.95 to 1.72; P = .11); in patients with centrally confirmed HER2-positive tumors, the HR was 1.47 (95% CI, 1.03 to 2.09; P = .03).
As first-line therapy for HER2-positive metastatic BC, lapatinib combined with taxane was associated with shorter PFS and more toxicity compared with trastuzumab combined with taxane.