Abstract
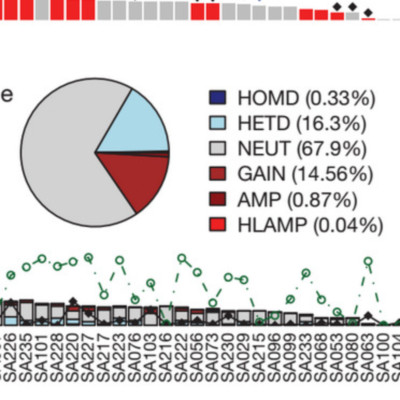
Primary triple-negative breast cancers (TNBCs), a tumour type defined by lack of oestrogen receptor, progesterone receptor and ERBB2 gene amplification, represent approximately 16% of all breast cancers. Here we show in 104 TNBC cases that at the time of diagnosis these cancers exhibit a wide and continuous spectrum of genomic evolution, with some having only a handful of coding somatic aberrations in a few pathways, whereas others contain hundreds of coding somatic mutations. High-throughput RNA sequencing (RNA-seq) revealed that only approximately 36% of mutations are expressed. Using deep re-sequencing measurements of allelic abundance for 2,414 somatic mutations, we determine for the first time-to our knowledge-in an epithelial tumour subtype, the relative abundance of clonal frequencies among cases representative of the population. We show that TNBCs vary widely in their clonal frequencies at the time of diagnosis, with the basal subtype of TNBC showing more variation than non-basal TNBC. Although p53 (also known as TP53), PIK3CA and PTEN somatic mutations seem to be clonally dominant compared to other genes, in some tumours their clonal frequencies are incompatible with founder status. Mutations in cytoskeletal, cell shape and motility proteins occurred at lower clonal frequencies, suggesting that they occurred later during tumour progression. Taken together, our results show that understanding the biology and therapeutic responses of patients with TNBC will require the determination of individual tumour clonal genotypes.