Abstract
Germline truncating mutations in DICER1, an endoribonuclease in the RNase III family that is essential for processing microRNAs, have been observed in families with the pleuropulmonary blastoma-family tumor and dysplasia syndrome. Mutation carriers are at risk for nonepithelial ovarian tumors, notably sex cord-stromal tumors.
We sequenced the whole transcriptomes or exomes of 14 nonepithelial ovarian tumors and noted closely clustered mutations in the region of DICER1 encoding the RNase IIIb domain of DICER1 in four samples. We then sequenced this region of DICER1 in additional ovarian tumors and in certain other tumors and queried the effect of the mutations on the enzymatic activity of DICER1 using in vitro RNA cleavage assays.
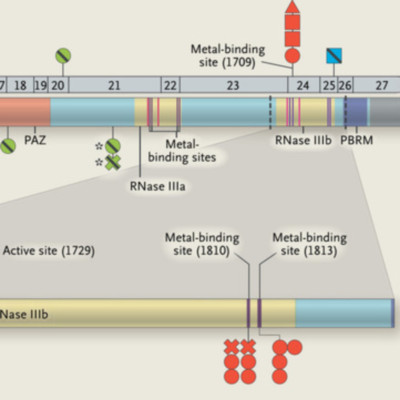
DICER1 mutations in the RNase IIIb domain were found in 30 of 102 nonepithelial ovarian tumors (29%), predominantly in Sertoli-Leydig cell tumors (26 of 43, or 60%), including 4 tumors with additional germline DICER1 mutations. These mutations were restricted to codons encoding metal-binding sites within the RNase IIIb catalytic centers, which are critical for microRNA interaction and cleavage, and were somatic in all 16 samples in which germline DNA was available for testing. We also detected mutations in 1 of 14 nonseminomatous testicular germ-cell tumors, in 2 of 5 embryonal rhabdomyosarcomas, and in 1 of 266 epithelial ovarian and endometrial carcinomas. The mutant DICER1 proteins had reduced RNase IIIb activity but retained RNase IIIa activity.
Somatic missense mutations affecting the RNase IIIb domain of DICER1 are common in nonepithelial ovarian tumors. These mutations do not obliterate DICER1 function but alter it in specific cell types, a novel mechanism through which perturbation of microRNA processing may be oncogenic. (Funded by the Terry Fox Research Institute and others.).