Abstract
Mll5 is currently a member of the Mll family of SET domain histone methyltransferase proteins but studies have also showed that it could be part of the SET3 branch of proteins. Recently, constitutive knock out animal studies have shown that Mll5 is required for proper haematopoietic stem cell differentiation, and loss of Mll5 results in synthetic lethality for genome de-methylation. Mll5 deficient male mice are infertile and here we analyse the consequences of Mll5 deficiency for spermatogenesis.
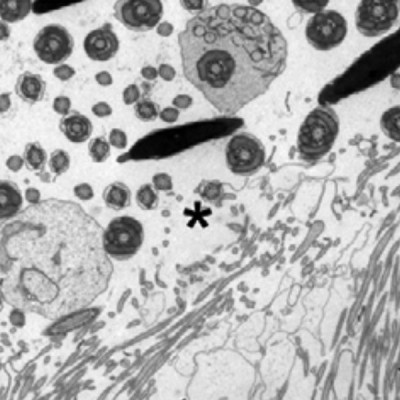
Mll5 deficient male mice, but not female mice, are infertile. Here we show using RNA in-situ hybridization that Mll5 is expressed in the germ cells of the testes of wild type mice. Consistent with the expression of Mll5, we demonstrate by electron microscopy, video microscopy and in vitro fertilisation techniques that Mll5 deficient mice have defects in terminal maturation and packaging of sperm. The defects seen include detachment of the acrosomal cap and impaired excess cytoplasm removal. Functional tests of sperm motility show a lack of progressive motility of spermatozoa from Mll5 deficient animals. None of these defects could be rescued by in vitro fertilization. Using microarray analysis we show that transcripts implicated in spermatogenesis are dysregulated.
Our data demonstrate a clear role of Mll5 in mammalian spermatogenesis at the level of terminal differentiation providing further support for its classification in the SET3 branch of proteins. Moreover, this study identifies Tlk2, Utx, Gpr64, Sult4a1, Rap2ip, Vstm2 and HoxA10 as possible Mll5 targets that together may account for the observed spermatozoa maturation defects.