Abstract
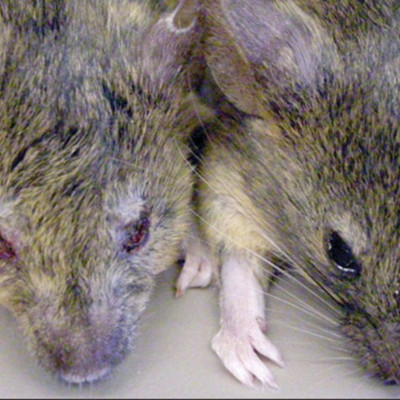
MLL5 is a divergent member of the Drosophila Trithorax-related (SET) domain and plant homeodomain (PHD) domain-containing chromatin regulators that are involved in the regulation of transcriptional “memory” during differentiation. Human MLL5 is located on chromosome 7q22, which frequently is deleted in myeloid leukemias, suggesting a possible role in hemopoiesis. To address this question, we generated a loss-of-function allele (Mll5(tm1Apa)) in the murine Mll5 locus. Unlike other Mll genes, Mll5(tm1Apa) homozygous mice are viable but display defects in immunity and hematopoiesis. First, Mll5(tm1Apa) homozygous mice show increased susceptibility to spontaneous eye infections, associated with a cell-autonomous impairment of neutrophil function. Second, Mll5(tm1Apa/tm1Apa) mice exhibit a mild impairment of erythropoiesis. Third, Mll5(tm1Apa/tm1Apa) hematopoietic stem cells (HSCs) have impaired competitive repopulating capacity both under normal conditions and when subjected to self-renewal stimulation by NUP98-HOXA10. Fourth, Mll5(tm1Apa) homozygous HSCs show a dramatic sensitivity to DNA demethylation-induced differentiation (5-azadeoxycytidine). Taken together, our data show that MLL5 is involved in terminal myeloid differentiation and the regulation of HSC self-renewal by a mechanism that involves DNA methylation. These data warrant investigation of MLL5 expression levels as a predictive marker of demethylating-agent response in patients with myelodysplastic syndromes and leukemias and identify MLL5 as a key regulator of normal hematopoiesis.