Abstract
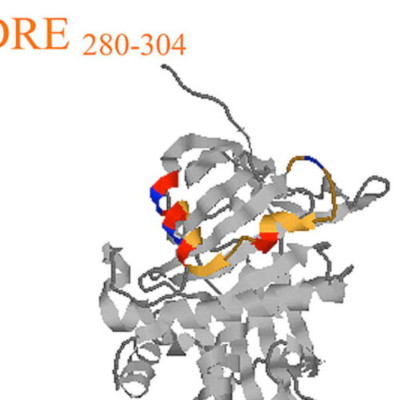
Pigment epithelium derived factor (PEDF) is non-inhibitory serpin with neurotrophic and antiangiogenic functions. In this study, we have assembled PEDF sequences for 9 additional species by data base mining and performed cross-species alignment for 14 PEDF sequences to identify conserved structural domains. We found evolutionary conservation of a leader sequence, a single C-terminal glycosylation site, collagen-binding residues, and four specific conserved PEDF peptides. The C-terminus, 384–415 and an N-terminal region 78–95, show close homology with many other serpins, and there is strong conservation of 39 of 51 consensus key residues involved in serpin structure and function. Two peptide regions, 40–67 and 277–301, are unique to PEDF but conserved in all species. Conserved residues at the N-terminus, helix d (hD), and helix A (hA) of PEDF form a structure similar to the heparin-binding groove of other serpins. We identified a motif in PEDF that is homologous to the nuclear localization signals of other proteins. A bitopographical localization of PEDF was confirmed by immunocytochemistry and Western blots. Our results suggest that secretion is required for PEDF’s activity, that PEDF can migrate to the nucleus, and that PEDF has structural and functional features more common with inhibitory serpins.